เมื่อฤดูหนาวมาถึงและอุณหภูมิลดลง ธรรมชาติก็จะมอบปรากฏการณ์ที่น่าหลงใหลให้กับเรา: เกล็ดหิมะ คริสตัลน้ำแข็งที่ละเอียดอ่อนและซับซ้อนเหล่านี้ไม่เพียงแต่เปลี่ยนโลกให้กลายเป็นดินแดนมหัศจรรย์ในฤดูหนาว แต่ยังดึงดูดจินตนาการของเราด้วยการออกแบบที่เป็นเอกลักษณ์และเฉพาะตัว คุณเคยสงสัยหรือไม่ว่าเกล็ดหิมะก่อตัวอย่างไรและทำไมแต่ละอันจึงแตกต่างกัน? ในโพสต์บล็อกนี้ เราจะสำรวจกระบวนการก่อตัวของเกล็ดหิมะที่น่าหลงใหลและเจาะลึกถึงเหตุผลทางวิทยาศาสตร์เบื้องหลังความเป็นเอกลักษณ์ของเกล็ดหิมะ
สิ่งที่จำเป็นสำหรับการก่อตัวของเกล็ดหิมะคืออะไร?
เกล็ดหิมะเป็นคริสตัลน้ำแข็งขนาดเล็กที่ก่อตัวขึ้นสูงในท้องฟ้า ซึ่งอากาศเย็นและชื้น ลักษณะของพวกมันขึ้นอยู่กับหลายปัจจัย เช่น อุณหภูมิ ความชื้น และสภาพอากาศ
- อุณหภูมิเป็นสิ่งสำคัญมากสำหรับการก่อตัวของเกล็ดหิมะ แล้วมันต้องเย็นแค่ไหนถึงจะมีหิมะตก? อากาศต้องเย็นกว่าจุดเยือกแข็ง (32°F หรือ 0°C) เพื่อให้น้ำระเหยกลายเป็นน้ำแข็ง เมื่ออากาศเย็นพอ โมเลกุลของไอน้ำจะช้าลงและเกาะติดกัน ทำให้เกิดคริสตัลน้ำแข็ง
- ความชื้นคือปริมาณไอน้ำในอากาศ ยิ่งอากาศชื้นมากเท่าไหร่ เกล็ดหิมะก็ยิ่งใหญ่และซับซ้อนมากขึ้นเท่านั้น ไอน้ำจะเกาะติดกับสิ่งเล็กๆ ในอากาศ เช่น ฝุ่นหรือละอองเกสร และสร้างแกนเล็กๆ ให้คริสตัลน้ำแข็งเติบโต
- สภาพอากาศยังส่งผลต่อการก่อตัวของเกล็ดหิมะอีกด้วย เมื่ออากาศสงบและมั่นคง คริสตัลน้ำแข็งสามารถเติบโตในรูปทรงที่ชัดเจนและสวยงาม เมื่ออากาศหยาบหรือเปลี่ยนแปลงอย่างรวดเร็ว คริสตัลน้ำแข็งจะเติบโตอย่างไม่สม่ำเสมอและมีรายละเอียดน้อยลง
เกล็ดหิมะก่อตัวอย่างไร
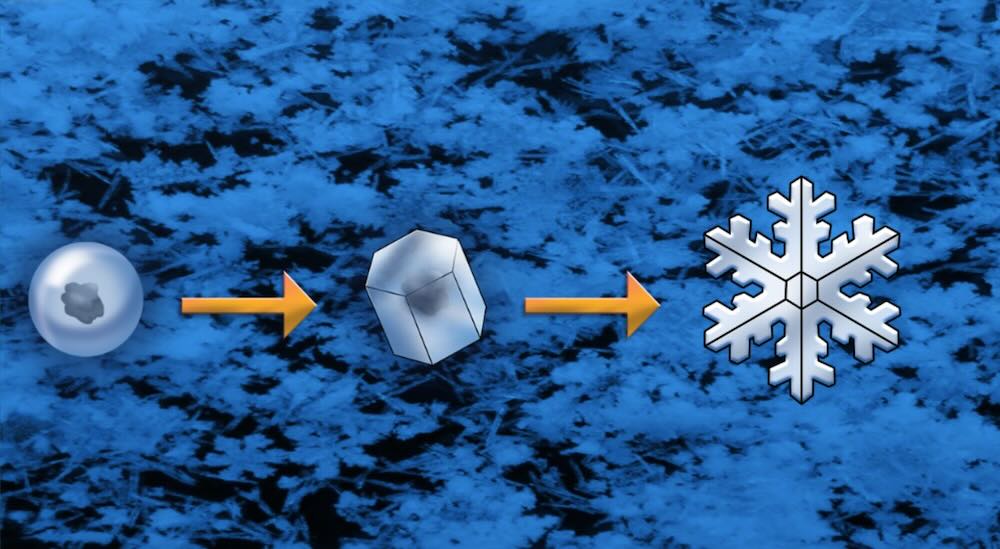
ในระหว่างการก่อตัว เกล็ดหิมะจะผ่านขั้นตอนสำคัญดังต่อไปนี้: การเกิดนิวเคลียส การเติบโต และการแตกแขนง
 แหล่งที่มาของภาพ: ABC57
แหล่งที่มาของภาพ: ABC57
กระบวนการเกิดนิวเคลียสในเกล็ดหิมะเป็นขั้นตอนแรกในการก่อตัวของคริสตัลน้ำแข็ง มันเกิดขึ้นเมื่อโมเลกุลของน้ำในบรรยากาศมารวมกันและจัดระเบียบตัวเองรอบอนุภาคเล็กๆ เช่น ฝุ่น ละอองเกสร หรือเกลือ อนุภาคเหล่านี้ทำหน้าที่เป็นแหล่งนิวเคลียส โดยให้พื้นผิวสำหรับโมเลกุลของน้ำในการยึดเกาะ หากไม่มีอนุภาคเหล่านี้ คริสตัลน้ำแข็งจะก่อตัวได้ยากขึ้น
หลังจากที่โมเลกุลของน้ำเกาะติดกับแหล่งนิวเคลียสแล้ว พวกมันจะเริ่มจัดเรียงตัวเองในโครงสร้างตาข่ายหกเหลี่ยม ความสมมาตรหกเหลี่ยมนี้เป็นผลมาจากโครงสร้างโมเลกุลของน้ำแข็ง เมื่อแช่แข็ง โมเลกุลของน้ำจะสร้างรากฐานสำหรับความสมมาตรหกด้านที่เรารู้จักกับเกล็ดหิมะ
แต่ละเกล็ดหิมะมีความแตกต่างกันเพราะมันเติบโตในรูปแบบต่างๆ ขึ้นอยู่กับสภาพอากาศ คริสตัลน้ำแข็งจะใหญ่ขึ้นเมื่อโมเลกุลของน้ำแข็งตัวบนมันมากขึ้น ทำให้เกิดชั้นหลายชั้น กระบวนการนี้เรียกว่าการสะสม ซึ่งโมเลกุลของน้ำในอากาศจะแข็งตัวบนคริสตัลที่มีอยู่แล้ว วิธีการเติบโตของคริสตัลเปลี่ยนแปลงไปตามอุณหภูมิและความชื้นของอากาศ บางครั้ง ส่วนต่างๆ ของคริสตัลเติบโตเร็วหรือช้ากว่าส่วนอื่นๆ ทำให้เกิดการแตกแขนง เกล็ดหิมะจะมีเอกลักษณ์มากขึ้นเรื่อยๆ เมื่อมันเติบโตและตกผ่านส่วนต่างๆ ของท้องฟ้า
รูปแบบเกล็ดหิมะที่เป็นเอกลักษณ์
วิลสัน เบนท์ลีย์ หรือที่รู้จักในชื่อ “ชายเกล็ดหิมะ” เป็นชาวไร่ในรัฐเวอร์มอนต์ที่อุทิศชีวิตให้กับการถ่ายภาพเกล็ดหิมะ ในช่วงปลายศตวรรษที่ 19 และต้นศตวรรษที่ 20 เบนท์ลีย์ถ่ายภาพเกล็ดหิมะแต่ละอันมากกว่า 5,000 ภาพภายใต้กล้องจุลทรรศน์ (ไมโครกราฟ) เผยให้เห็นโครงสร้างที่ซับซ้อนและเป็นเอกลักษณ์ของพวกมัน ผลงานของเบนท์ลีย์แสดงให้เห็นถึงความหลากหลายที่น่าทึ่งของรูปทรงเกล็ดหิมะ สมาคมประวัติศาสตร์เจริโคในเวอร์มอนต์เป็นที่ตั้งของพิพิธภัณฑ์เกล็ดหิมะเบนท์ลีย์ ซึ่งอุทิศให้กับการอนุรักษ์ผลงานและมรดกของวิลสัน เบนท์ลีย์ ผู้เข้าชมสามารถสำรวจไมโครกราฟเกล็ดหิมะต้นฉบับของเบนท์ลีย์และเรียนรู้เกี่ยวกับวิทยาศาสตร์การก่อตัวของเกล็ดหิมะ
 แหล่งที่มาของภาพ: Wikipedia
แหล่งที่มาของภาพ: Wikipedia
แล้วทำไมเกล็ดหิมะแต่ละอันถึงมีเอกลักษณ์? มาหาคำตอบกัน
สภาพบรรยากาศ
แม้ว่าพวกมันจะมีโครงสร้างหกเหลี่ยมร่วมกัน แต่เกล็ดหิมะแต่ละอันก็มีการเดินทางของตัวเอง ขณะที่มันตกลงสู่พื้น มันจะพบกับปัจจัยด้านสิ่งแวดล้อมมากมายที่กำหนดการเติบโตและการออกแบบของมัน จำนวนชุดค่าผสมและรูปแบบที่เป็นไปได้จำนวนมากทำให้มั่นใจได้ว่าไม่มีเกล็ดหิมะสองอันที่เหมือนกัน
องค์ประกอบของบรรยากาศ
องค์ประกอบของบรรยากาศยังส่งผลต่อการก่อตัวของเกล็ดหิมะอีกด้วย สิ่งเจือปนต่างๆ เช่น อนุภาคฝุ่นหรือมลพิษในบรรยากาศ สามารถมีอิทธิพลต่อการเติบโตของคริสตัลและเปลี่ยนรูปร่างสุดท้ายของเกล็ดหิมะ ปฏิสัมพันธ์ระหว่างคริสตัลกับสภาพแวดล้อมนี้เพิ่มความเป็นเอกลักษณ์อีกชั้นหนึ่งให้กับการออกแบบของเกล็ดหิมะแต่ละอัน
 แหล่งที่มาของภาพ: Tim Garrett, มหาวิทยาลัยยูทาห์, NSF.gov
แหล่งที่มาของภาพ: Tim Garrett, มหาวิทยาลัยยูทาห์, NSF.gov
การเย็นตัวเกิน
การเย็นตัวเกินเป็น ปรากฏการณ์ทางอากาศที่น่าหลงใหล ซึ่งมีบทบาทสำคัญในการกำหนดโครงสร้างเกล็ดหิมะที่หลากหลาย การเย็นตัวเกินเกิดขึ้นเมื่อน้ำยังคงอยู่ในสถานะของเหลวต่ำกว่าจุดเยือกแข็ง โดยทั่วไปเป็นเพราะขาดแหล่งนิวเคลียสในการเริ่มกระบวนการแข็งตัว เป็นผลให้หยดน้ำในบรรยากาศสามารถคงอยู่เป็นหยดน้ำที่เย็นตัวเกินได้แม้ในอุณหภูมิที่ต่ำกว่าจุดเยือกแข็ง
เมื่อคริสตัลน้ำแข็งพบกับหยดน้ำที่เย็นตัวเกิน มันจะทำหน้าที่เป็นแหล่งนิวเคลียส ทำให้หยดน้ำแข็งตัวทันทีเมื่อสัมผัส กระบวนการแช่แข็งอย่างรวดเร็วนี้จะกระตุ้นการเติบโตของคริสตัลน้ำแข็ง ซึ่งตอนนี้มีแพลตฟอร์มใหม่ให้ขยายออกไป แขนที่แตกแขนงและรายละเอียดที่ซับซ้อนของเกล็ดหิมะมักพัฒนาผ่านกระบวนการเย็นตัวเกินนี้ การพบกับน้ำที่เย็นตัวเกินแต่ละครั้งทำให้คริสตัลเติบโตอย่างมีเอกลักษณ์และไม่สมมาตร
สัณฐานวิทยาของเกล็ดหิมะ: ประเภทของคริสตัลน้ำแข็ง
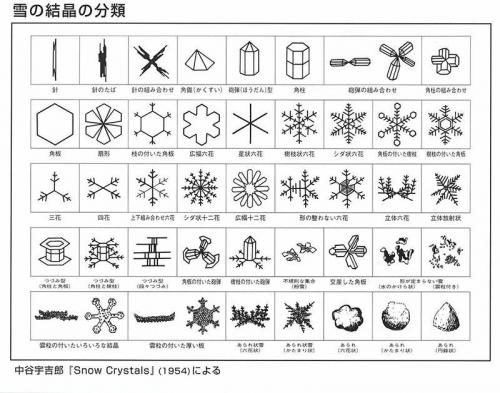
ในช่วงต้นศตวรรษที่ 20 Ukichiro Nakaya นักฟิสิกส์ชาวญี่ปุ่นได้ทำการวิจัยอย่างกว้างขวางเกี่ยวกับคริสตัลน้ำแข็งและพัฒนาระบบการจำแนกประเภทตามรูปร่างของพวกมัน เขายังเป็นคนแรกในโลกที่สร้างหิมะเทียมในปี 1936 ผลการค้นพบของเขาถูกนำเสนอในพิพิธภัณฑ์หิมะและน้ำแข็ง Nakaya Ukichiro ในเมืองคากะ บ้านเกิดของนักวิทยาศาสตร์
 แหล่งที่มาของภาพ: Kaga General Services, Japan Experience
แหล่งที่มาของภาพ: Kaga General Services, Japan Experience
เราได้สรุปประเภทของคริสตัลน้ำแข็งที่พบบ่อยที่สุดในตารางด้านล่าง
| รูปร่าง | คำอธิบาย |
|---|---|
| เดนไดรต์ | เกล็ดหิมะแบบคลาสสิกที่มีโครงสร้างหกกิ่งคล้ายดาว มักมีขนาดใหญ่และมีรายละเอียดซับซ้อน |
| คอลัมน์ | เกล็ดหิมะทรงกระบอกยาวที่มีด้านหกเหลี่ยมตามความยาว |
| แผ่น | เกล็ดหิมะแบนหกเหลี่ยมที่มีการแตกแขนงจำกัด คล้ายกับแผ่นบาง |
| เข็ม | เกล็ดหิมะยาวเรียวที่มีรูปร่างคล้ายเข็ม |
| เดนไดรต์คอลัมน์ | การรวมกันของรูปแบบคอลัมน์และเดนไดรต์ มีคอลัมน์กลางที่มีแขนคล้ายกิ่ง |
| คอลัมน์ที่มีฝาปิด | เกล็ดหิมะทรงกระบอกที่มีปลายแบนคล้ายจาน |
| กลุ่มที่ไม่สม่ำเสมอ | กลุ่มของคริสตัลหิมะที่มีรูปร่างไม่สม่ำเสมอและรวมตัวกันแบบสุ่ม |
| เกล็ดหิมะที่มีรอยน้ำแข็ง | เกล็ดหิมะที่มีหยดน้ำแข็งตัว (รอยน้ำแข็ง) บนพื้นผิว |
| เกล็ดหิมะที่ผ่านกระบวนการรอยน้ำแข็ง | เกล็ดหิมะที่ผ่านกระบวนการรอยน้ำแข็งและกลายเป็นเม็ดน้ำแข็งกลม |
ทำไมเกล็ดหิมะถึงมีหกด้านและข้อเท็จจริงที่น่าสนใจอื่นๆ
เกล็ดหิมะมีหกด้านเนื่องจากวิธีการสร้างน้ำแข็ง โมเลกุลของน้ำแต่ละโมเลกุลมีออกซิเจนหนึ่งอะตอมและไฮโดรเจนสองอะตอม ซึ่งมีลักษณะคล้ายตัว V เมื่อแช่แข็ง โมเลกุลเหล่านี้จะเกาะติดกันในลักษณะที่ทำให้เกิดหกด้าน
นี่คือข้อเท็จจริงที่น่าสนใจอื่นๆ เกี่ยวกับเกล็ดหิมะ:
- เกล็ดหิมะที่ใหญ่ที่สุด ตามบันทึกของ Guinness World Records เกล็ดหิมะที่ใหญ่ที่สุดที่เคยบันทึกไว้ตกลงมาในช่วงพายุหิมะในฟอร์ตคีโอห์ รัฐมอนแทนา สหรัฐอเมริกา เมื่อวันที่ 28 มกราคม พ.ศ. 2430 เกล็ดหิมะขนาดมหึมานี้มีเส้นผ่านศูนย์กลางประมาณ 15 นิ้ว (38.1 ซม.)
- เกล็ดหิมะสิบสองด้าน แม้ว่าเกล็ดหิมะหกด้านจะพบได้บ่อยที่สุด แต่เกล็ดหิมะที่มีด้านมากกว่านั้นสามารถเกิดขึ้นได้ภายใต้เงื่อนไขบางประการ ตัวอย่างเช่น ในเดือนธันวาคม 2022 ช่างภาพจากโคโลราโดได้ถ่ายภาพระยะใกล้ของเกล็ดหิมะสิบสองด้านที่สวยงาม
 แหล่งที่มาของภาพ: Jason Persoff
แหล่งที่มาของภาพ: Jason Persoff
เกล็ดหิมะฝุ่นเพชร ภายใต้สภาพบรรยากาศบางอย่าง โดยเฉพาะอย่างยิ่งในอุณหภูมิที่เย็นจัดและความชื้นต่ำมาก ปรากฏการณ์ที่หายากที่เรียกว่า “ฝุ่นเพชร” สามารถเกิดขึ้นได้ มันเกี่ยวข้องกับการก่อตัวของคริสตัลน้ำแข็งขนาดเล็กในอากาศ สร้างภาพลวงตาของอนุภาคคล้ายเพชรที่ลอยอยู่ในท้องฟ้า
เกล็ดหิมะในอวกาศ ในปี 2016 ดาวอายุน้อยชื่อ V883 Orionis มีวงแหวนของหิมะรอบๆ นักวิทยาศาสตร์ค้นพบมันโดยใช้กล้องโทรทรรศน์ ALMA หิมะในอวกาศเกิดขึ้นเมื่อน้ำซึ่งอยู่ในรูปของก๊าซอยู่ไกลจากดาวมากเกินไปและกลายเป็นน้ำแข็ง ก๊าซน้ำมักจะแข็งตัวใกล้กับดาวมากกว่าก๊าซอื่นๆ ดังนั้นจึงมองเห็นได้ยากจากโลกเพราะดาวสว่างเกินไป แต่ V883 Orionis มีการระเบิดของความร้อนอย่างกะทันหันที่ผลักเส้นหิมะออกไปไกลกว่าเดิม – ประมาณเท่ากับระยะทางจากดาวพลูโตถึงดวงอาทิตย์ สิ่งนี้ทำให้นักวิทยาศาสตร์มองเห็นหิมะได้ง่ายขึ้น
 แหล่งที่มาของภาพ: NRAO • ALMA
แหล่งที่มาของภาพ: NRAO • ALMA
บทสรุป
เกล็ดหิมะเป็นสิ่งมหัศจรรย์ที่แท้จริงของธรรมชาติ ทำให้เราหลงใหลด้วยความงามของพวกมัน การทำความเข้าใจกระบวนการก่อตัวของเกล็ดหิมะทำให้เราสามารถชื่นชมความเป็นเอกลักษณ์ของพวกมันได้มากขึ้น เมื่อคริสตัลน้ำแข็งตกลงมาจากท้องฟ้า สภาพบรรยากาศที่เปลี่ยนแปลงตลอดเวลาจะส่งผลต่อการเติบโตของพวกมัน ส่งผลให้เกิดการออกแบบที่น่าหลงใหล เกล็ดหิมะแต่ละอันแสดงถึงการเดินทางที่ไม่เหมือนใครผ่านบรรยากาศ ซึ่งแสดงถึงความซับซ้อนและความหลากหลายของธรรมชาติของเรา ดังนั้น ครั้งต่อไปที่คุณพบกับหิมะตก ใช้เวลาสักครู่เพื่อชื่นชมผลงานชิ้นเอกที่ซับซ้อนซึ่งเป็นเกล็ดหิมะ ```






